Abstract
Background: Fludarabine, cyclophosphamide and rituximab (FCR) regimen was previously recommended as standard chemoimmunotherapy for young fit patients (pts) with CLL prior to the advent of novel target drugs. Orelabrutinib, a highly selective BTK inhibitor, had approved for relapsed/refractory (RR) CLL treatment. Obinutuzumab, a glycoengineeredtype II CD20 mAb, had shown to be superior to rituximab in CLL treatment.Pts achieving minimal residual disease (MRD)-negative status in peripheral blood (PB) or bone marrow (BM) had a better progression free survival (PFS) and overall survival (OS) outcome irrespective of treatment received compared to those who could not achieve MRD negativity (CRISTALLO endorsed).
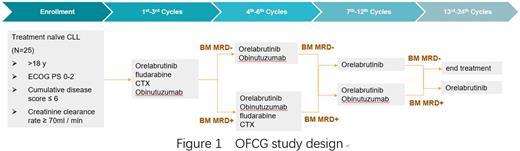
Methods: This is an investigator-initiated phase II trial (NCT 05322733). Eligible pts had previously untreated CLL/SLL with ECOG PS 0-2, 18-65 years old, Cumulative disease score ≤ 6, and Creatinine clearance rate ≥ 70 ml / min (N=25). Patients with IGHV unmutated, del (17p) and/or TP53 Mutation are included.
Enrolled patients will receive 7-day lead-in therapy of Orelabrutinib 150 mg once daily. Then all Pts receive three cycles of OFCG. After the 3 cycles, following evaluation by MRD in BM detected by multi-color flow cytometry (FCM) at 10-4 sensitivity according to ERIC methodology. Patients will continue to receive 3 cycles of OFCG regimen if the MRD is positive. Otherwise, the patients will continue to receive 3 cycles of OG regimen if the MRD is negative. At the 7th-12th cycles, Based on the achievement of uMRD in BM at the end of Cycle 6, patients would be decided to received O monotherapy (with uMRD) or OG regimen (without uMRD). Similarly, at the 13rd-24th cycles, patients would be decided whether to discontinue treatment based on the evaluation results of Cycle 12. (Figure 1)
We set every 28 days as a dosing cycle. Obinutuzumab, 1000mg, D1/D8/D15 in C1, D1 in other cycles, (first dosing: 100 mg in D1 and 900 mg in D2). Orelabrutinib: 150 mg, QD. Fludarabine: 25 mg/m2, D1-3, per cycle. CTX: 250 mg/m2, D1-3, per cycle.
The primary endpoint is the percentage of undetectable MRD in BM after 6 cycles of treatment. The percentage of MRD negativity by NGS is an exploratory endpoint (at 10-6 sensitivity).
Results:
By the end of July 2022, 13 patients were screened, of which 7 patients have begun treatment with the combination regimen, and 1 patient completed 2 cycles treatment and the first efficacy evaluation who with undetectable BM MRD after 3 cycles treatment. We will update the first efficacy evaluation reporting of 10 patients at this ASH.
Conclusions: Enrollment, treatment and follow up continues.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Orelabrutinib, a Highly Selective BTK Inhibitor, had approved for RR CLL treatment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal